The SMARTer Human TCR a/b Profiling Kit enables users to analyze T-cell receptor (TCR) diversity from single T cells that have been sorted into a 96-well plate. As the name suggests, the kit can be used to generate data for both alpha- and beta-chain diversity. The kit leverages SMART technology and employs a 5' RACE-like approach to capture complete V(D)J variable regions of TCR transcripts. Included in the kit are primers that incorporate Illumina-specific adaptor sequences during cDNA amplification. The protocol generates indexed libraries that are ready for sequencing on Illumina platforms.
- Human TCR repertoire analysis in single cells (TCR-alpha and TCR-beta subunits)
- TCR alpha-beta pairing determination in single cells
Why single-cell TCR analysis is needed
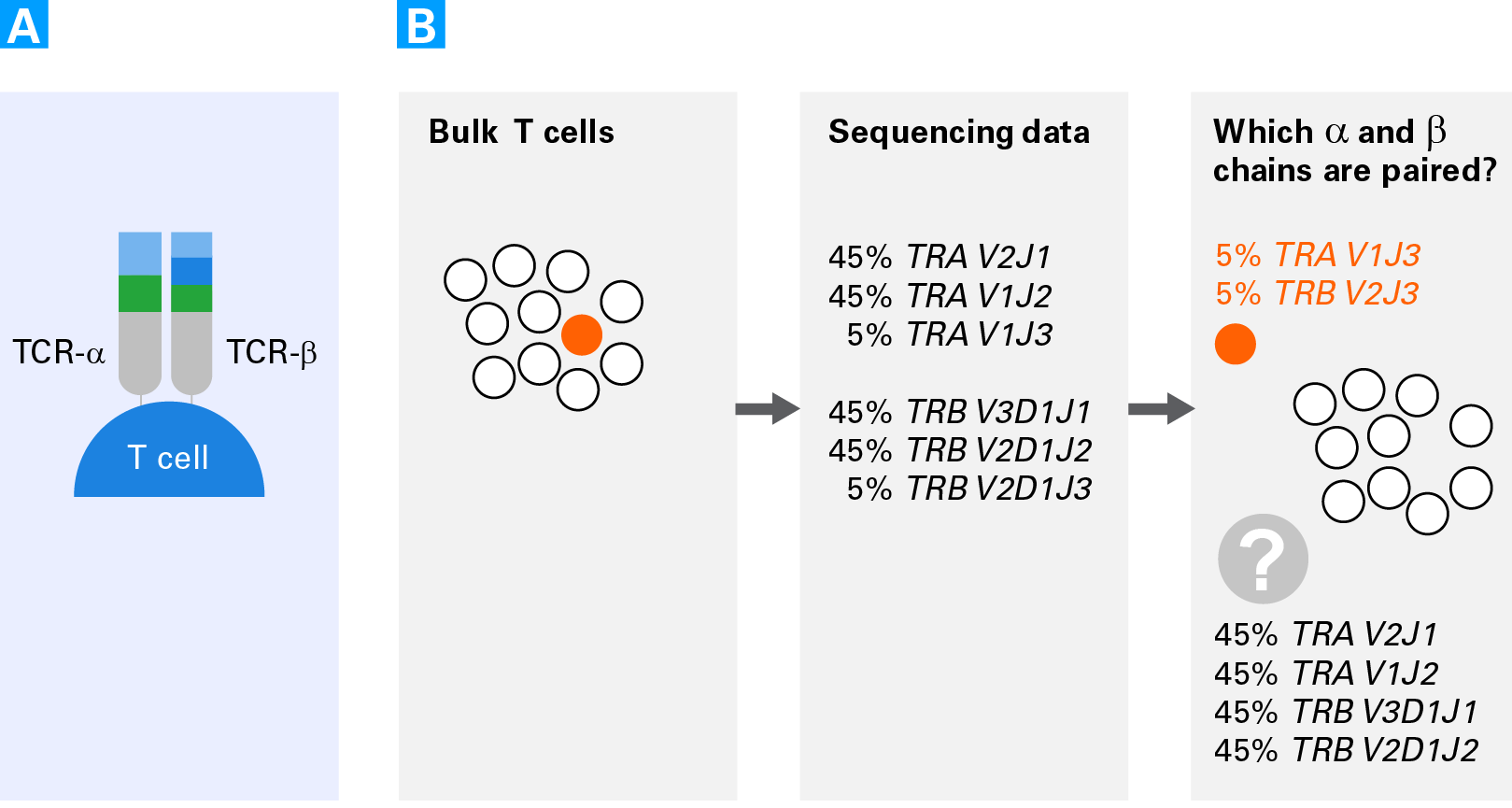
Benefits of studying T cells at the single-cell level. Panel A. Schematic of a T-cell receptor comprised of an alpha chain (TCR-α) and a beta chain (TCR-β). Panel B. Schematic representing the difficulties of obtaining pairing information for alpha and beta chains from bulk sequencing data. Rare clonotypes can allow for assessing the pairing of some cells (clonotypes in orange). However, for the more highly expressed clonotypes (TCRA V2J1, TCRA V1J2, TCRB V3D1J1, TCRB V2D1J2) there are four possible pairing combinations.
Bioanalyzer traces from example TCR libraries
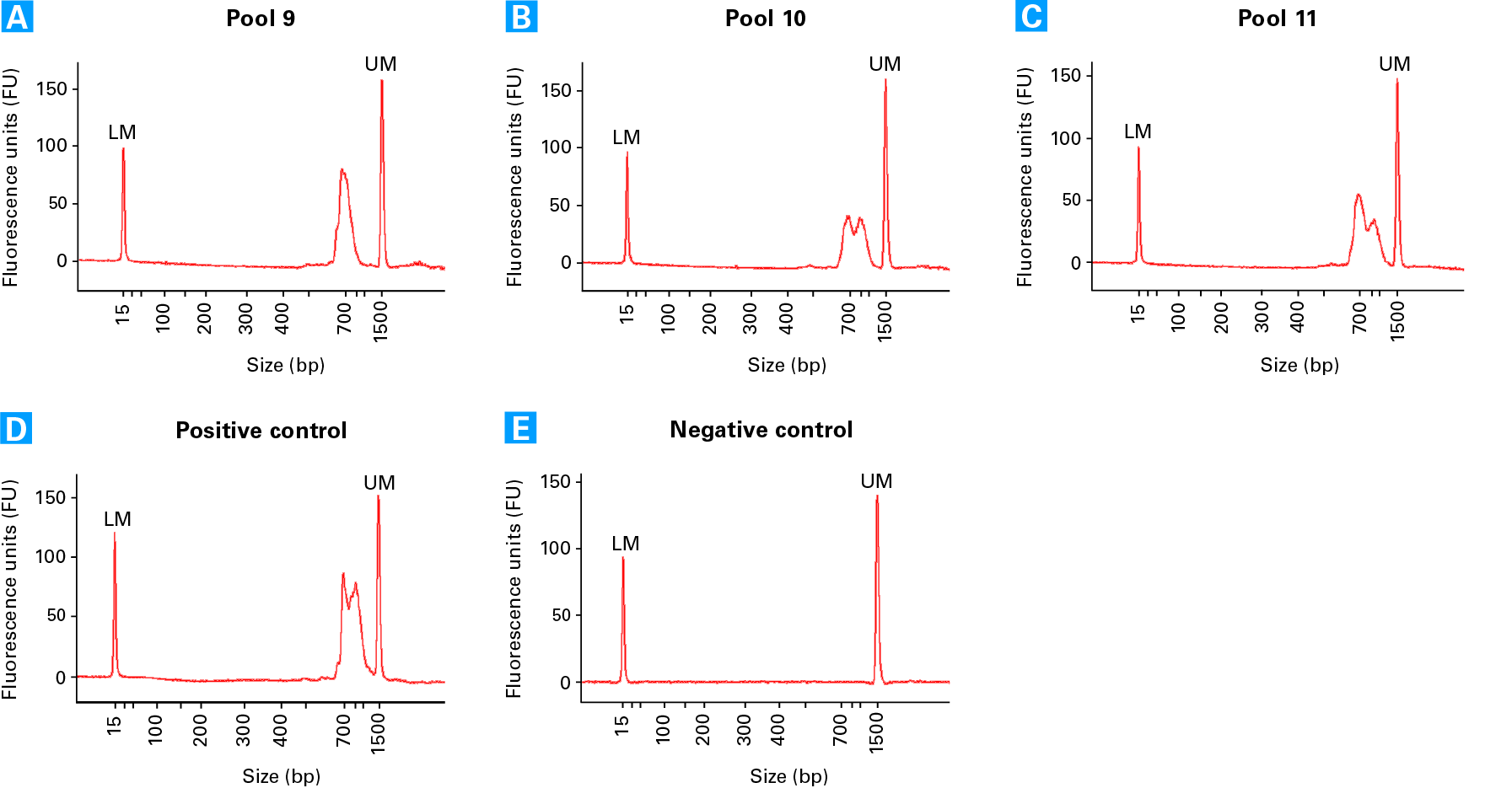
Electropherogram profiles of TCR sequencing libraries. Electropherograms from three different cell pools (see technical note for more details): Pool 9 (Panel A), 10 (Panel B), and 11 (Panel C). The variation in the profiles reflects the abundance of amplified TCRa (peak at ~900 bp) and TCRb (peak at ~700 bp). Panel D. Positive Control RNA TCR library (generated with 8 x 5 pg Control Jurkat Total RNA). Panel E. Negative Control TCR library shows no library is produced.
The SMARTer scTCR workflow uses optimized indexing to allow for pooling 96 cells into 12 libraries.
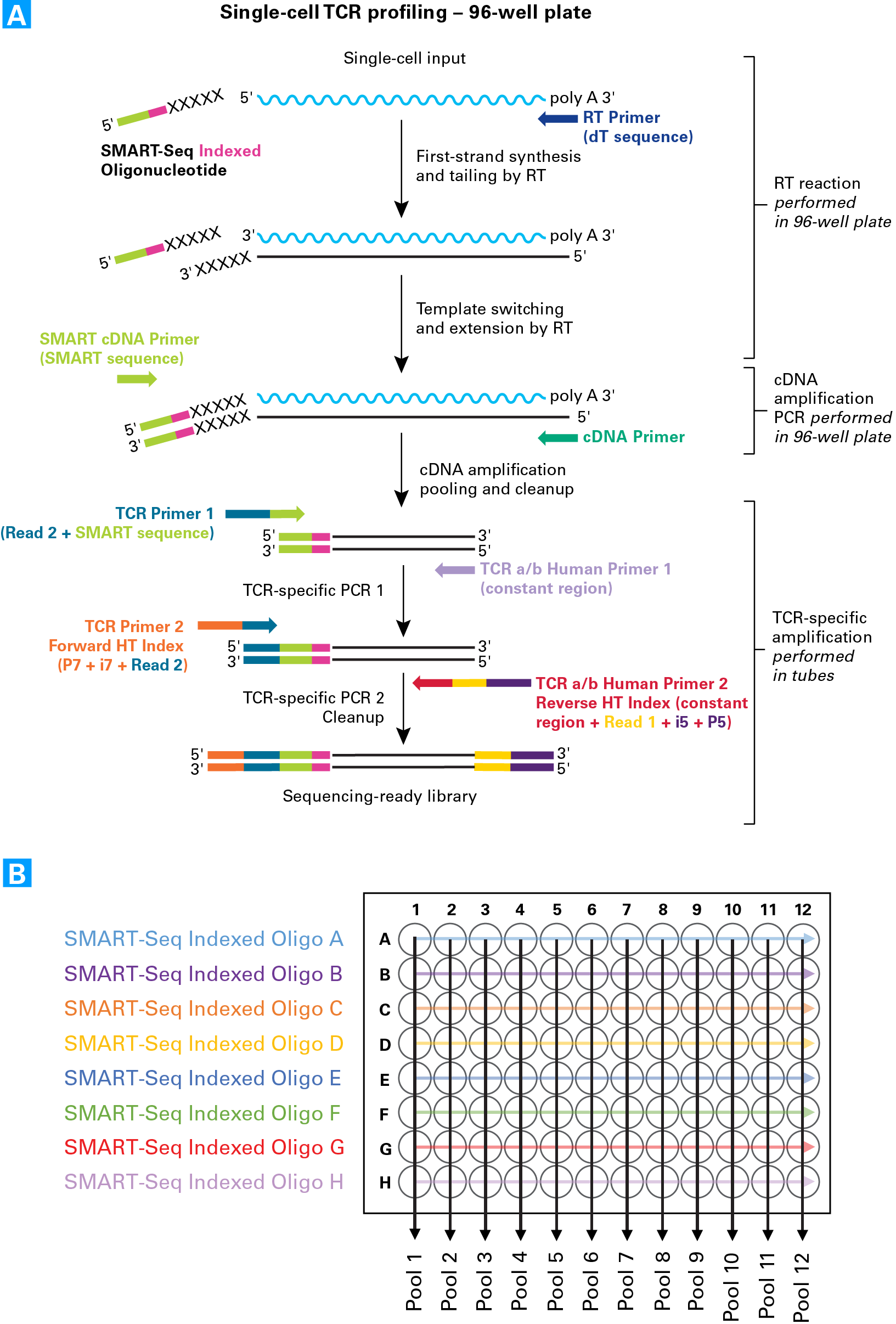
SMARTer Human scTCR a/b Profiling Kit workflow and pooling strategy. Panel A. First-strand cDNA synthesis is dT-primed (RT Primer) and performed by an MMLV-derived reverse transcriptase (RT), which adds nontemplated nucleotides to the 5' end of each mRNA template. The SMART-Seq Indexed Oligos anneal to these nontemplated nucleotides and serve as a template for the incorporation of an additional sequence of nucleotides into the first-strand cDNA by the RT (this is the template-switching step). Each of the eight different SMART-Seq Indexed Oligos provided in the kit each contains a unique six-base in-line index that serves as a cell barcode to allow downstream cell identification after pooling. The additional sequence added to the cDNA by the RT—referred to as the "SMART sequence"—serves as a primer-annealing site for subsequent rounds of PCR, ensuring that only sequences from full-length cDNAs undergo amplification. After pooling (described in Panel B) and a cleanup step, two rounds of gene-specific PCR are performed in succession to amplify cDNA sequences corresponding to variable regions of TCRa and/or TCRb transcripts. The first gene-specific PCR uses the amplified double-stranded cDNA as a template and includes a forward primer with complementarity to the SMART sequence—which also incorporates the Illumina Read 2 sequence (TCR Primer 1)—and reverse primers that are complementary to the constant (i.e., nonvariable) region of TCRa and TCRb (TCR a/b Human Primer 1). The second round of PCR takes the product from the first PCR as a template and uses a forward primer that binds to the Read 2 sequence added by the previous PCR step. The reverse primers bind in the constant region, internal to the PCR1 primers (TCR a/b Human Primer 2 Reverse HT Index) allowing amplification of the entire variable region and a portion of the constant region of TCRa and TCRb cDNA. The forward and reverse primers include adapter and index sequences that are compatible with the Illumina sequencing platform and allow for multiplexing of up to 96 samples in a single flow-cell lane. Panel B. Samples are pooled by column, such that each pool contains eight cells each with a differently indexed SMART-Seq Indexed Oligo. Different combinations of the Forward and Reverse HT indexes are used during PCR 2 to allow multiplexing of the samples into a single flow-cell lane (see the User Manual for more details).
 Menu
Menu