An important update is available for FreeStyle LibreLinkØ. Check here for more information.
 Menu
Menu
27 Sep 2018
Flow cytometry is a laser-based technology widely used in biological research, from fundamental science to large scale clinical studies alike. Flow cytometers are typically used to enumerate and examine microscopic particles, such as cells or other small vesicles. This makes it an efficient and quantitative method for analysing cells or particles in suspension. As well as being able to interrogate cells for their physical characteristics (e.g. size and granularity) fluorescent probes, such as fluorescently tagged antibodies, can be used to profile cell surface and intracellular proteins or components. In addition, florescent dyes can be used to label cellular components allowing various metabolic properties of cells to be assessed, such as mitochondrial health, cell cycle and viability.
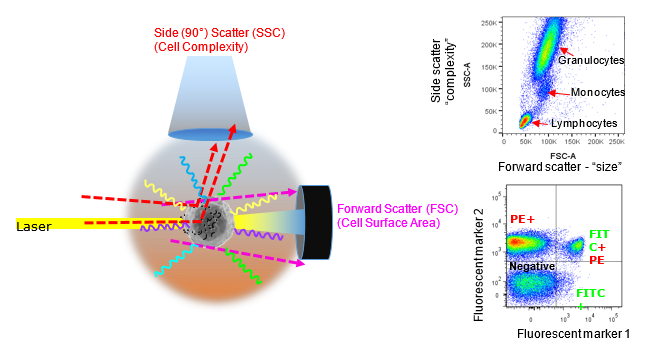
Forward versus side scatter (FSC vs SSC) gating is commonly indicated to characterise cell size and complexity or granularity, however, this is a slight oversimplification. In any case, the light scattering properties can be useful to at least identify cell populations of interest. The better known feature of a flow cytometer is fluorescence detection. As particles (cells) pass through a focused light source the emission of fluorescent labels can be measured. Image courtesy of Dr Anna Brooks.
Flow cytometry has often been regarded as an immunologist’s playground, and accordingly, flow cytometers will most often be found in or associated with immunology research labs. However, flow cytometry core facilities (or shared resource laboratories) are a mainstay of many if not all scientific institutes. Many aspects of cell function, physiology or components of cells can be measured using cytometric techniques, and therefore researchers from a wide range of disciplines can find themselves in the driver’s seat of a cytometer. Just as long as a fluorescent probe, such as an antibody or dye is available to target your cell or component of interest, the types of analyses that can be performed on cytometers are virtually limitless. Many different cell types can be analysed, including mammalian, bacterial, protozoan, yeast and plant, even reaching down to the level of microvesicles and subcellular components. In addition, there are many functional assays that can be performed to assess various characteristics such as cell-cell interactions, cell secretions, viability, proliferation and killing.
Flow cytometers can be either cell analysers, cell sorters, or both. Flow cytometry analysers can be used to enumerate and profile cells, whereas cell sorters can also be used to not only measure cell characteristic’s, but also physically separate live cell populations of interest for further downstream analyses. Being able to isolate single cells or populations of viable cells for further characterisation is immensely powerful, especially when multiple cell surface markers may be needed to identify or exclude contaminating cells. Indeed, the field of single cell transcriptomics was entirely dependent on cell sorters for isolating individual cells until microfluidic devices entered the market. Whether for analyses or to isolate interesting cell populations, flow cytometry is a powerful technique for dissecting heterogeneity by allowing the rapid measurement of multiple parameters, simultaneously, in a complex single cell suspension.
There are many types of fluorescent probes that enable cell characterisation by flow cytometry. From fluorescent proteins found in nature (eg Phycoerythrin (PE), allophycocyanin (APC), GFP, YFP) to synthetic compounds (eg FITC, Texas Red, Alexa 488), there are many dyes that have been utilised for their fluorescent detection properties. These are often conjugated to primary antibodies or alternatively stain or bind directly to proteins or subcellular components (eg DNA dyes). As well as natural or synthetic fluorescent compounds, chemically combined dyes have allowed further expansion of the colour palette available for utilisation in multicolour flow cytometry. These so-called tandem dyes are composed of two distinct fluorophores that are chemically linked (coupled) close together to create a distinct spectral excitation and emission profile. Although these dyes expand the range of colours that can be used off each laser, they do have some disadvantages that one must be aware of when utilising them in multicolour applications.
Over the last decade there has been an explosion in technological advances which has seen an expansion in the number of commercially available fluorescent dyes tagged to antibodies, and instrumentation capable of detecting over 18 colours simultaneously. Applying high dimensionality (18+ colours) to precious or limited samples is now in high demand and is driving innovation in the field. For many years multicolour analyses were limited by reagent availability, and therefore only experienced research groups with the resources to in-house conjugate reagents were performing multicolour, multiparametric or “polychromatic” analyses (Perfetto et al, 2004). The release of Sirigen polymer dyes and their associated tandems transformed multicolour flow cytometry by offering a new range of reagents in previously un-tapped spectral ranges. These new dyes not only opened up the colour palate, they also aimed to maximise the spectral detection space from each laser line. These dyes include the Brilliant Violet™ Brilliant UltraVioletTM, and Brilliant BlueTM options. However, just as new dyes were expanding multicolour capabilities, researchers were quick to fill every available channel on their cytometers, further driving demand for more colours, more detectors, and more complexity.
Next came the drive for instrumentation innovation. Conventional flow cytometer designs sought to meet the multicolour requirements by adding more lasers and detectors, which significantly increases the cost of ownership, maintenance and usage. However, more recently, innovations in optical detection have seen a new wave of instruments enter the market. One such new-generation flow cytometer, the Cytek AuroraTM, is a spectral analyser that represents a new level of capability for complex multicolour flow cytometry. However, as this technology is the new-kid on the block and an apparent game-changer in the multicolour arena, we will stick to ‘all-things conventional” to avoid confusion. As the complexity of flow cytometers increases, so too does the need for ensuring that best practices in flow cytometric techniques are followed. Data acquired should not only be accurate, but also reproducible. Flow cytometry, unfortunately, is one of those techniques where errors are often committed “unknowingly”, therefore education on best practice is paramount. There are now loads of great resources available from published literature, online resources and instrument/reagent vendors, so there really is no excuse for committing flow cytometric crimes!
References
Perfetto, S. P., et al. (2004). "Seventeen-colour flow cytometry: unravelling the immune system." Nature Reviews Immunology 4: 648.
Dr Anna Brooks holds a BCA (management) and a PhD in Immunology and is a Senior Research Fellow with the Maurice Wilkins Centre at the University of Auckland. Anna is also an expert flow cytometrist and is director of Auckland Cytometry, the flow cytometry core facility for the Faculty of Science. Anna’s primary interest lies in developing multicolour panels to characterise complex cellular populations in digested human tissues. Anna is also an active member of the international flow cytometry community and currently sits on the Australasian Cytometry Society Council as research liaison. When not marvelling in the many fluorescent colours of the cytometry world, Anna enjoys to scuba dive and explore the vibrant wonders of our underwater world.
If you enjoyed reading our articles, why not sign up to our blog mailing list? You'll get new articles straight to your inbox as they're released!